Last decades, a fuel cell has received huge attention as a net-zero carbon emission solution. Particularly, the automotive industry responded quickly to developing fuel cell electric vehicles to overcome the environmental challenges of internal combustion engine vehicles. South Korean automotive company, Hyundai Motor is one of the market leaders in the hydrogen-powered fuel-cell car industry (Figure 1). In this article, a basic concept of hydrogen full cell will be explained including its manufacturing process, benefits and current limitations.

The Concept of fuel cell
A fuel cell is an electrochemical cell that converts chemical energy, often hydrogen, to electricity. In Figure 2, the basic concept of the fuel cell core part, Membrane Electrode Assembly (MEA) is shown. Hydrogen is provided to the anode side and Oxygen is supplied to the cathode side. The anode panel is coated with a catalyst, mostly platinum powder and it splits hydrogen into electrons and protons. The protons freely travel through the designed electrolyte and at the same time electrons are forced to travel through an electric circuit. During the travelling, electrons carry energy and it produces direct current electricity. When electrons arrive at the cathode side, another catalyst, often nickel causes electrons, protons and oxygen to react, forming water which will release through a vape pipe to outside. This process will continue until the continuous hydrogen and oxygen supplies are stopped.

The components of a fuel cell and how to build it
To build a fuel cell, some important factors need to be considered, for example, power output, materials, size, operating conditions etc.
First of all, the output needs to be decided. The output voltage of a single cell is normally designed to have 0.6-0.7 V output at nominal power [1]. As most applications require higher voltage and power, a fuel cell stack that connects multiple individual cells in series is often used for industrial applications. The number of cells in a stack needs to be decided as well based on the required voltage and power. The power output can be determined by calculating the highest possible voltage spike and adding a safety factor [1].
The single fuel cell consists of multiple layers like a sandwich. These components are nicely stacked and clamped together (figure 3). Some commercial fuel cell stacks also contain cooling and humidification systems.
(End Plate)
↓
(Current Collector & Gaskets)
↓
(Flowfield Plate)
↓
(Membrane Electrode Assembly (MEA) & Gasket)
Catalyst - Gas Diffusion Layer (GDL) - Electrolyte - GDL - Catalyst
↓
(Flowfield Plate)
↓
(Current collector & Gaskets)
↓
(End Plate)
Figure 3. Assembly order of single fuel cell stack is explained.
On the endplates, there is a Hydrogen inlet and outlet. To avoid any fuel or oxidant (often air or oxygen) leaking between the layers, gaskets and spacers are used (figure 4).

The next step is selecting materials for each fuel cell component. Different types of the fuel cell are made use of different materials. In this article, one of the popular types of fuel cells, Proton Exchange Membrane Fuel Cell (PEMFC) will be used to explain the basic structure of fuel cell.
For PEMFC, usually, platinum is used for the catalyst as it is the most effective material to split oxygen molecules into protons and electrons. Additionally Platinum is the only metal that can withstand the acidic conditions inside such a cell and speed up chemical reactions [2]. The catalyst layer is made with several methods such as painting, sputter diffusion, mechanical deposition and also the easiest/cheapest method, screen printing [1].
Regarding the polymer electrolyte membrane part, Nafion® is the most common material [1].
A Gas Diffusion Layer (GDL) is often made with a conductive carbon fibre cloth or paper and it is bonded to the catalyst. The conductive carbon fibre cloth is often coated with Teflon on one side to help with the water management in the fuel cell stack [1].
The Benefits and Challenges of hydrogen fuel cells
The hydrogen fuel cell is promising for the net-zero carbon emission solution. It can reduce greenhouse gas emissions because it only produces water vapour through the process. Additionally, hydrogen can be made from renewable energy such as sunlight, wind and water. As there is no environmental impact during the hydrogen fuel cell operation, it has been widely used in different industries such as heavy-duty material handling, stationary and transportation. Furthermore, in Europe, toward the net-zero emissions scenarios for 2050, many new policies have been introduced to support hydrogen applications (figure 5).
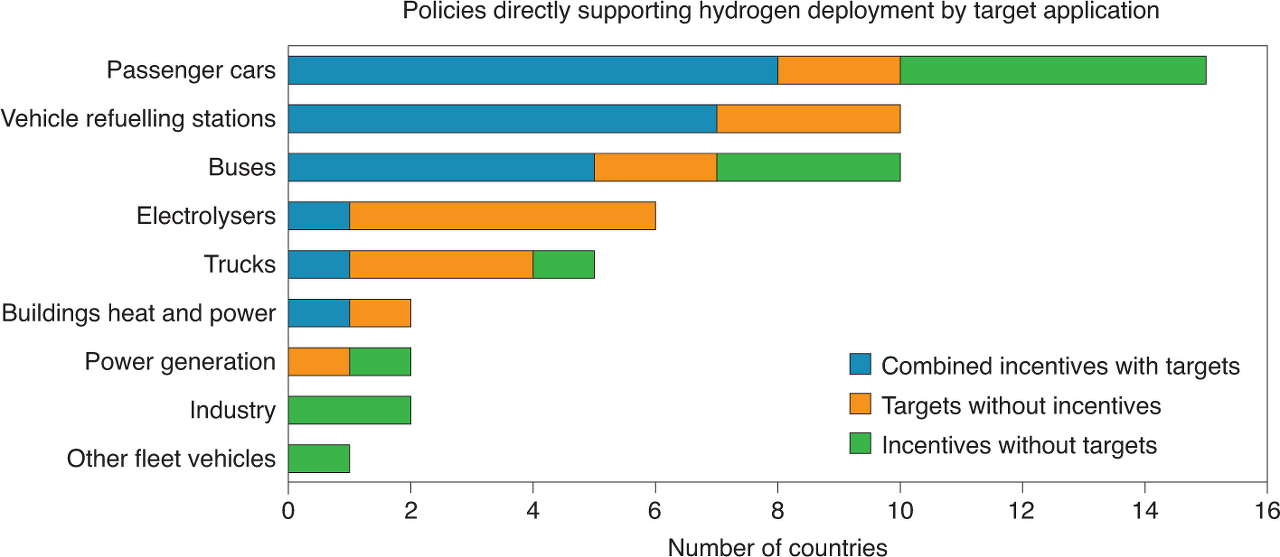
Nevertheless the notable advantages of fuel cells, there are also remained hurdles to jump over.
First of all, the high costs of materials and manufacturing technologies are key factors to slow down the commercial usage of hydrogen fuel cells [3]. The main material for catalysts is platinum which is one of the most precious metals on the planet. Many companies have developed alternative materials to replace platinum, however, due to its high efficiency, still, it is the most popular catalyst for the fuel cell. Due to its high cost, the production of hydrogen also cannot satisfy the increasing demands from the industries [4].
More importantly, the benefit of the net-zero carbon emission relies entirely on how hydrogen is produced. There are colour codes to categorized hydrogen by production methods. 'Green hydrogen' is made from the electrolysis of water, therefore it's a carbon emission-free energy solution. Yet, the majority of hydrogen is produced from natural gas and the process emits CO2. It's named 'Grey hydrogen'. Grey hydrogen production process is cheaper than green hydrogen's [4], so for companies, it is not easy to choose Green hydrogen over Grey hydrogen cost-wise.
[Reference]
[1] C. Spiegel, "How to Build a Fuel Cell", Fuelcellstore.com, 2018. [Online]. Available: https://www.fuelcellstore.com/blog-section/how-to-build-a-fuel-cell. [Accessed: 22- Dec- 2021].
[2] P. Patel, "A Better Platinum Catalyst for Fuel Cells", MITTechnologyReview, 2010. [Online]. Available: https://www.technologyreview.com/2010/05/05/203948/a-better-platinum-catalyst-for-fuel-cells/#:~:text=A%20good%20catalyst%20should%20be,weakly%20with%20the%20oxygen%20atoms. [Accessed: 22- Dec- 2021].
[3] A. Alaswad, "Technical and Commercial Challenges of Proton-Exchange Membrane (PEM) Fuel Cells", Energies, 2021, vol 14, no 1, pp. 144.
[4] S van Renssen, "The Hydrogen Solution?", Nature Climate Change, 2020, vol 10, no 9, 799-801.
'Technology > Fuel Cells' 카테고리의 다른 글
| Gas Diffusion Layer (GDL) (0) | 2022.05.24 |
|---|
